When The Quanity Of A Subsatanve Increased What Doesnt Change?
Lesson Overview for Teachers
View the video below to see what you and your students will practice in this lesson.
Objective
Students will be able to make measurements showing that whether the process is a change of state, dissolving, or a chemic reaction, the total mass of the substances does not alter.
Annotation: In the demonstrations and activities in this lesson, substances volition exist weighed earlier and later on diverse processes have occurred – either melting, dissolving, or a chemical reaction. The bones principle students should observe and conclude is that mass is conserved in these processes, so the mass should non change. Students may notice slight variations of plus or minus 0.i grams, depending on the sensitivity of the balance or whether the mass is actually somewhere between two values. If there is a minor modify in mass, explain to students that small differences may be caused by a slight lack of precision in the scale readout, or past errors in the weighing methods, just that the overall results advise that mass is conserved in all of these processes.
Key Concepts
- When a substance changes state, the mass of the substance does non change.
- When a substance dissolves in a liquid, the total mass of the substance and the liquid it dissolves in does not change.
- When substances react to form new substances as products, the mass of the products is the same every bit the mass of the reactants.
NGSS Alignment
- NGSS 5-PS1-2:Mensurate and graph quantities to provide evidence that regardless of the blazon of change that occurs when heating, cooling, or mixing substances, the total weight of matter is conserved.
Note: In this lesson, students measure and observe that mass is conserved during the processes of melting, dissolving, and chemical modify. The students volition non make a graph.
Summary
- Students cheque to meet whether the mass of ice and water in a cup changes equally the water ice melts.
- Students also test whether the combined mass of sugar and water changes after sugar is dissolved in the h2o.
- As a demonstration, students will observe that a precipitate forms in a reaction betwixt solutions of magnesium sulfate and sodium carbonate, and that the mass of the products is the same as the mass of the reactants.
Evaluation
Download the student activity sheet (PDF) and distribute one per student when specified in the activity. The activity canvass will serve as the Evaluate component of the 5-E lesson programme.
Safe
Brand sure you and your students wear properly plumbing fixtures safety goggles. Sodium carbonate may cause peel and serious eye irritation. Follow all safety precautions regarding the use, storage, and disposal of sodium carbonate.
Make clean-upwards and Disposal
Remind students to wash their easily after completing the action. All mutual household or classroom materials can exist saved or disposed of in the usual fashion.
Materials
Materials needed for each group
- 1 Articulate plastic cup
- H2o
- 1 Teaspoon of sugar
Materials for the Engage sit-in
- 1 Clear plastic loving cup
- H2o
- 1 Ice cube
- Scale
Materials for the EXTEND demonstration
- 2 Clear plastic cups
- Sodium carbonate
- Magnesium sulfate (Epsom salt)
- Water
- Graduated cylinder
- Teaspoon
Engage
1.Do a demonstration to prove that melting ice in h2o does non crusade the mass of the combined water and ice to change.
Question to investigate: Volition the combined mass of water and ice stay the same as the ice in the cup melts?

Procedure:
- Cascade water into a articulate plastic cup then that it is near 1/3-full.
- Add one piece of ice.
Inquire students:
- If we weigh this loving cup with the water and ice, do y'all call back the combined mass volition modify as the ice melts?
No. - Why or why not?
Because the ice is just melting. It is all the same the same amount of h2o, but it's only irresolute from a solid to a liquid. Information technology should accept the aforementioned mass.
Annotation: It is possible that some water may evaporate from the cup as the water ice melts, causing the contents of the cup to weigh a little less at the end of the process. On the other hand, any water condensing on the exterior of the cup could make it weigh a fiddling more. Neither of these factors is likely to contribute much to the combined mass measurements, since very little water will evaporate or condense in the time it takes for the ice to cook.

- Weigh the cup, water, and water ice. Record the combined mass on the activity sheet.
While the ice melts, have students bear the experiment below. When they are washed with that experiment and the ice has melted, prove students the mass of the water and melted water ice.
Expected results
The mass should be the aforementioned.
Give each pupil an Activity Sheet (PDF).
Students will record their observations and answer questions about the activity on the activity sheet.
Explore
2. Have students counterbalance water and sugar before and after the sugar dissolves.
Question to investigate: Will the combined mass of sugar and water be the same after the carbohydrate dissolves in the h2o?
Inquire students:
- If y'all weigh a loving cup of water and a teaspoon of sugar and then dissolve the sugar in the water, practise you recollect the mass volition change?
No.
- Why or why not?
Because the same corporeality of sugar is however there. The solid saccharide crystals break apart in water equally the sugar dissolves, but the individual carbohydrate particles or molecules are still nowadays and do non alter as a consequence of dissolving in the water. The combined mass of the saccharide and water shouldn't modify.

Materials for each group:
- 1 Articulate plastic cup
- Water
- 1 Teaspoon of sugar
Procedure
- Add together h2o to the loving cup until it is near 1/4-full.
- Add 1 teaspoon of sugar to the h2o.
- Weigh the cup with the water and sugar and record the mass.
Annotation: Evaporating water could brand the h2o and sugar weigh a footling less. This will probably not be a cistron since very piddling water will evaporate in the time it takes for the sugar to deliquesce.

- Carefully swirl the cup to assist the sugar dissolve.
- When the saccharide is dissolved, identify the cup back on the scale to measure the mass.
Expected results
The combined mass does non change.
Ask students:
- Did the mass change?
No.
iii. Do a sit-in to see if the mass changes during a chemical reaction.
Question to investigate: Volition the mass change when reactants combine to class products in a chemical reaction?
Materials for the sit-in
- 2 Clear plastic cups
- Sodium carbonate
- Magnesium sulfate (Epsom salt)
- Water
- Graduated cylinder
- Teaspoon

Expected results
A white solid will form. At first the solid may announced or look like clouds of white particles floating in the liquid, but the particles should eventually settle out to course a solid precipitate at the bottom of the loving cup.
Tell students that a chemical reaction took place and that a new substance, a solid, was formed.
Ask students:
- Do you recollect the total mass of the two cups, the combined solutions, with the white solid will be more, less or the same as it was before the reaction took place?
Same.

- Identify both cups on the scale to measure out the total mass.
Expected results
The full mass should exist the same equally the sum of the individual masses recorded before the contents of the cups were combined and the reaction took place.
Explain that the reactants have been transformed into a new substance, but that all the individual atoms making up the reactants are nonetheless present in the products. That'south why the mass stays the same.
Explain
4. Show an blitheness to assistance explain why mass is conserved in melting, dissolving, and in a chemical change.

Bear witness the animation Conservation of Mass in Concrete and Chemical Changes.
Explicate that whether a process involves melting, dissolving, or a chemical reaction, all the atoms that were at that place earlier the process takes place are still there after any changes take occurred, and so the overall mass stays the same.
Extend
5. Evidence an animation to help explicate why mass is conserved when water is frozen.
Remind students that they observed that the overall mass of water and ice stayed the same as the ice melted.
Ask students:
- When water freezes to form ice, it takes up more room in the container, but does its mass modify?
Even though the volume of water changes every bit it becomes ice, the mass of the h2o should remain the same before and later on it turns into water ice.
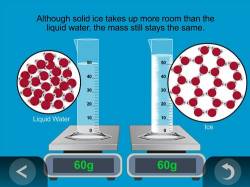
Evidence the blitheness Mass is Conserved in Freezing.
Explain that even though water takes upward more than room when frozen, the same number of h2o molecules are still there so the mass stays the same.
Source: https://www.acs.org/content/acs/en/education/resources/k-8/inquiryinaction/fifth-grade/chapter-4/conservation-of-mass.html
Posted by: steeleconswited1948.blogspot.com

0 Response to "When The Quanity Of A Subsatanve Increased What Doesnt Change?"
Post a Comment